
The molecules of the substance haven't changed at all. When the matter goes through a chemical change, it can’t return to its original state without additional reactions whereas when it goes through a physical change, it only needs to return to its original state of matter. Physical Change: It is a process in which a substance changes its state of matter, but chemical bonds stay intact. The basic definitions of chemical and physical changes are given below:Ĭhemical Change: It is a process in which chemical bonds are broken or created to make a new substance. The primary difference between a chemical change and a physical change is what happens to a substance’s composition. For instance, bleaching a stain, adding vinegar to baking soda, fermenting grapes, etc. Once the chemical alteration takes place, it cannot be overturned. Energy change is one of the features of a chemical change, because of the development of the new product. The evolution of energy, the creation of bubbles, and changes in temperature are some signs of chemical change.Īlternately, known as a chemical reaction, where the substances involved are called reactants, and the result of the reaction is known as a product. When a substance experiences a chemical change, the chemical character of the substance changes and it is converted into a different substance with a different chemical configuration. For instance, melting of wax, dissolving sugar in water, boiling of water, chopping wood, crumpling of paper, etc.Ĭhemical Change is defined as the procedure in which the atoms of one or more substances are reordered or combined to form a new substance. the original physical appearance of the object remains unchanged. The same element or compound occurs prior, or after the change, i.e. These alterations are unstable that can be reversed using simple physical methods. solid, liquid, gas), etc., that, without making any alteration in their molecular composition. Physical change is a process in which the material experiences alteration in its physical properties like shape, size, volume, appearance, color, state (i.e.
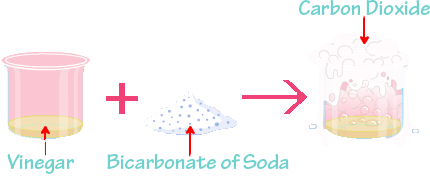
On the other hand, a chemical change is one that changes the internal structure of the substance, so as to proceed with a new substance. Physical changes are the changes that change the physical characters of the material, without making any change in their interior structure.

All these changes that take place nearby are chemical changes or physical changes. In our everyday life, we come across many changes in our surroundings, for example, souring of milk, rusting of iron, bread becoming toast, stretching of a rubber band, melting of wax, lilting of a match stick, etc. Changes can be classified as physical or chemical changes. Just as chemists have categorized elements and compounds, they have also classified kinds of changes. Change is occurring all around us all of the time.


 0 kommentar(er)
0 kommentar(er)
